
SL Paper 1
Which value of q, in J, has the correct number of significant figures?
q = mcΔT
where m = 2.500 g, c = 4.18 J g−1 K−1 and ΔT = 0.60 K.
A. 6
B. 6.3
C. 6.27
D. 6.270
Which molecule has an index of hydrogen deficiency (IHD) = 1?
A. C6H6B. C2Cl2
C. C4H9N
D. C2H6O
What is the ratio of the areas of the signals in the 1H NMR spectrum of pentan-3-ol?
A. 6:4:1:1
B. 6:2:2:2
C. 5:5:1:1
D. 3:3:2:2:1:1
Which feature of a molecule does infrared spectrometry detect?
A. molecular mass
B. bonds present
C. total number of protons
D. total number of proton environments
What is the index of hydrogen deficiency (IHD) for this molecule?
A. 3
B. 4
C. 5
D. 6
The enthalpy of combustion of ethanol is determined by heating a known mass of tap water in a glass beaker with a flame of burning ethanol.
Which will lead to the greatest error in the final result?
A. Assuming the density of tap water is 1.0 g cm−3
B. Assuming all the energy from the combustion will heat the water
C. Assuming the specific heat capacity of the tap water is 4.18 J g−1 K−1
D. Assuming the specific heat capacity of the beaker is negligible
What can be deduced from the following 1H\(\,\)NMR spectrum?
A. There is only one hydrogen atom in the molecule.
B. There is only one hydrogen environment in the molecule.
C. The molecule is a hydrocarbon.
D. There is only one isotope in the element.
What is the Index of Hydrogen Deficiency (IHD) for 1,3,5-hexatriene (C6H8)?
A. 1
B. 3
C. 5
D. 6
What can be determined about a molecule from the number of signals in its 1H\(\,\)NMR spectrum?
A. Bonds present
B. Molecular formula
C. Molecular mass
D. Number of hydrogen environments
Which information can be gained from an infrared (IR) spectrum?
A. Ionization energy of the most abundant element
B. Number of different elements in the compound
C. Bonds present in a molecule
D. Molecular formula of the compound
The graph below represents the relationship between two variables in a fixed amount of gas.
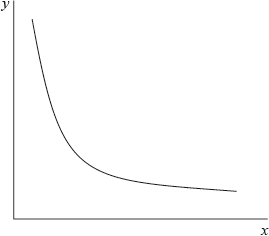
Which variables could be represented by each axis?
Which are likely to be reduced when an experiment is repeated a number of times?
A. Random errors
B. Systematic errors
C. Both random and systematic errors
D. Neither random nor systematic errors
What is the relationship between the two variables sketched on the graph?
A. y is proportional to x
C. y is proportional to −x
D. y decreases exponentially with an increase in x
A measuring cylinder was used to obtain a known volume of a liquid. The volume was read from the top of the meniscus and the liquid completely emptied into a flask. The exact same process was then repeated. Which statement is correct about the overall described procedure and the volumes measured?
B. There is a random error and the volumes measured are accurate.
C. There is a random error and the volumes measured are inaccurate.
D. There is a systematic error and the volumes measured are inaccurate.
A student recorded the volume of a gas as \({\text{0.01450 d}}{{\text{m}}^{\text{3}}}\). How many significant figures are there in this value?
A. 3
B. 4
C. 5
D. 6
The relationship between the pressure, \(P\), and the volume, \(V\), of a fixed amount of gas at a constant temperature is investigated experimentally. Which statements are correct?
I. A graph of \(V\) against \(P\) will be a curve (non-linear).
II. A graph of \(V\) against \(\frac{1}{P}\) will be linear.
III. \(V = {\text{constant}} \times \frac{{\text{1}}}{P}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
A burette reading is recorded as \(27.70 \pm 0.05{\text{ c}}{{\text{m}}^{\text{3}}}\). Which of the following could be the actual value?
I. \({\text{27.68 c}}{{\text{m}}^{\text{3}}}\)
II. \({\text{27.78 c}}{{\text{m}}^{\text{3}}}\)
III. \({\text{27.74 c}}{{\text{m}}^{\text{3}}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
What is the density, in g\(\,\)cm−3, of a 34.79 g sample with a volume of 12.5 cm3?
A. 0.359
B. 0.36
C. 2.783
D. 2.78
Which feature of a molecule can be determined from its 1H NMR spectrum?
A. Number of hydrogen environmentsB. Total mass of hydrogen atoms present
C. Vibration frequency of C–H bonds
D. Ionization energy of a hydrogen atom
What is always correct about the molecular ion, M+, in a mass spectrum of a compound?
A. The M+ ion peak has the smallest m/z ratio in the mass spectrum.
B. The m/z ratio of the M+ ion peak gives the relative molecular mass of the molecule.
C. The M+ ion is the most stable fragment formed during electron bombardment.
D. The M+ ion peak has the greatest intensity in the mass spectrum.
How many significant figures are there in 0.00370?
A. 2
B. 3
C. 5
D. 6
A piece of metallic aluminium with a mass of 10.044 g was found to have a volume of \({\text{3.70 c}}{{\text{m}}^{\text{3}}}\). A student carried out the following calculation to determine the density.
\[{\text{Density (g}}\,{\text{c}}{{\text{m}}^{ - 3}}{\text{)}} = \frac{{10.044}}{{3.70}}\]
What is the best value the student could report for the density of aluminium?
A. \({\text{2.715 g}}\,{\text{c}}{{\text{m}}^{ - 3}}\)
B. \({\text{2.7 g}}\,{\text{c}}{{\text{m}}^{ - 3}}\)
C. \({\text{2.71 g}}\,{\text{c}}{{\text{m}}^{ - 3}}\)
D. \({\text{2.7146 g}}\,{\text{c}}{{\text{m}}^{ - 3}}\)
Which would be the best method to decrease the random uncertainty of a measurement in an acid-base titration?
A. Repeat the titration
B. Ensure your eye is at the same height as the meniscus when reading from the burette
C. Use a different burette
D. Use a different indicator for the titration
A student weighs a standard 70.00 g mass five times using the same balance. Each time she obtains a reading of 71.20 g. Which statement is correct about the precision and accuracy of the measurements?
A. Precise and accurate
B. Precise but inaccurate
C. Accurate but not precise
D. Neither accurate nor precise
Ultraviolet radiation has a shorter wavelength than infrared radiation. How does the frequency and energy of ultraviolet radiation compare with infrared radiation?
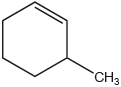
What is the index of hydrogen deficiency, IHD, of 3-methylcyclohexene?
A. 0
B. 1
C. 2
D. 3
How are the uncertainties of two quantities combined when the quantities are multiplied together?
A. Uncertainties are added.
B. % uncertainties are multiplied.
C. Uncertainties are multiplied.
D. % uncertainties are added.
What information is provided by 1H NMR, MS and IR for an organic compound?
I. 1H NMR: chemical environment(s) of protons
II. MS: fragmentation pattern
III. IR: types of functional group
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Which would be the best method to decrease the random uncertainty of a measurement in an acid–base titration?
A. Ensure your eye is at the same height as the meniscus when reading the burette.
B. Use a different indicator for the titration.
C. Use a different burette.
D. Repeat the titration.
In an experiment to determine a specific quantity, a student calculated that her experimental uncertainty was 0.9% and her experimental error was 3.5%. Which statement is correct?
A. Only random uncertainties are present in this experiment.
B. Both random uncertainties and systematic errors are present in this experiment.
C. Repeats of this experiment would reduce the systematic errors.
D. Repeats of this experiment would reduce both systematic errors and random uncertainties.
A student measured the change in mass on heating a sample of calcium carbonate, CaCO3(s). What is the mass loss?
Mass before heating: 2.347 g ± 0.001
Mass after heating: 2.001 g ± 0.001
A. 0.346g ± 0.001
B. 0.346g ± 0.002
C. 0.35g ± 0.002
D. 0.35g ± 0.001
A student heated a solid in a crucible. The student measured the mass of the solid and crucible before and after heating and recorded the results.
\[\begin{array}{*{20}{l}} {{\text{Mass of crucible and solid before heating}}}&{ = 101.692{\text{ g}}} \\ {{\text{Mass of crucible and solid after heating}}}&{ = 89.312{\text{ g}}} \end{array}\]
What value should the student record for the mass lost in grams?
A. 12.4
B. 12.38
C. 12.380
D. 12.3800
A student measured the mass and volume of a piece of silver and recorded the following values.

Which value, in \({\text{g}}\,{\text{c}}{{\text{m}}^{ - 3}}\), for the density of silver should the student report in her laboratory notebook?
A. 10.49
B. 10.4900
C. 10.5
D. 10.500
What is the best way to minimize the random uncertainty when titrating an acid of unknown strength against a standard solution of sodium hydroxide (ie one of known concentration)?
A. First standardize the sodium hydroxide solution against a standard solution of a different acid.
B. Use a pH meter rather than an indicator to determine the equivalence point.
C. Keep your eye at the same height as the meniscus when reading the burette.
D. Repeat the titration several times.
Density can be calculated by dividing mass by volume. \(0.20 \pm 0.02{\text{ g}}\) of a metal has a volume of \(0.050 \pm 0.005{\text{ c}}{{\text{m}}^{\text{3}}}\). How should its density be recorded using this data?
A. \(4.0 \pm 0.025{\text{ g}}\,{\text{c}}{{\text{m}}^{ - 3}}\)
B. \(4.0 \pm 0.8{\text{ g}}\,{\text{c}}{{\text{m}}^{ - 3}}\)
C. \(4.00 \pm 0.025{\text{ g}}\,{\text{c}}{{\text{m}}^{ - 3}}\)
D. \(4.00 \pm 0.8{\text{ g}}\,{\text{c}}{{\text{m}}^{ - 3}}\)
What is the graphical relationship between n and T in the ideal gas equation, pV = nRT, all other variables remaining constant?
A student carries out a titration three times and obtains the following volumes: \(3.0 \pm 0.1{\text{ c}}{{\text{m}}^3}\), \(3.2 \pm 0.1{\text{ c}}{{\text{m}}^3}\) and \(3.2 \pm 0.1{\text{ c}}{{\text{m}}^3}\). What is the average volume?
A. \(3.1 \pm 0.1{\text{ c}}{{\text{m}}^3}\)
B. \(3.13 \pm 0.1{\text{ c}}{{\text{m}}^3}\)
C. \(3.1 \pm 0.3{\text{ c}}{{\text{m}}^3}\)
D. \(3.13 \pm 0.3{\text{ c}}{{\text{m}}^3}\)
The heat change in a neutralization reaction can be determined by mixing equal volumes of HCl(aq) and NaOH(aq) of the same concentration in a glass beaker. The maximum temperature change is recorded using an alcohol thermometer.
What is the biggest source of error in this experiment?
A. Heat absorbed by the glass thermometer
B. Random error in the thermometer reading
C. Heat loss to the surroundings
D. Systematic error in measuring the volumes of HCl(aq) and NaOH(aq) using burettes
Which statement about errors is correct?
A. A random error is always expressed as a percentage.
B. A systematic error can be reduced by taking more readings.
C. A systematic error is always expressed as a percentage.
D. A random error can be reduced by taking more readings.
A student performs an acid-base titration using a pH meter, but forgets to calibrate it. Which type of error will occur and how will it affect the quality of the measurements?
A. Random error and lower precision
B. Systematic error and lower accuracy
C. Systematic error and lower precision
D. Random error and lower accuracy
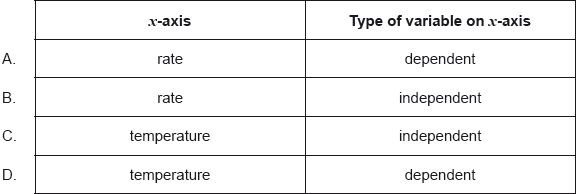
The rate of a reaction is studied at different temperatures.
Which is the best way to plot the data?
\({\text{50 c}}{{\text{m}}^{\text{3}}}\) of copper(II) sulfate solution is measured into a plastic cup using a \({\text{100 c}}{{\text{m}}^{\text{3}}}\) measuring cylinder. Excess zinc powder is added and the temperature rise that occurs is measured with a –10 °C to +110 °C thermometer. The enthalpy change for the reaction is then calculated. Which statement is correct?
A. Systematic error will be reduced by repeating the experiment several times and averaging the results.
B. Random error will be reduced by insulating the plastic cup.
C. Random error will be reduced by using a 50 cm\(^3\) graduated pipette instead of a measuring cylinder.
D. Systematic error will be increased by using a larger volume of copper(II) sulfate solution.
Graph 1 shows a plot of volume of CO2(g) against time for the reaction of CaCO3(s) with 1.00 moldm−3HCl (aq). The acid is the limiting reagent and entirely covers the lumps of CaCO3(s).
Which set of conditions is most likely to give the data plotted in graph 2 when the same mass of CaCO3(s) is reacted with the same volume of HCl(aq) at the same temperature?